Which Statement Best Describes an Oxidation Reduction Reaction
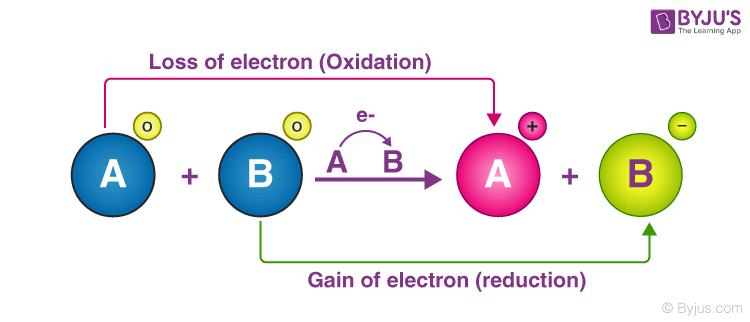
An oxidation-reduction redox reaction is a type of chemical reaction in which electrons are transferred between chemical species. You might be interested in.

Chemistry Reduction And Oxidation Reactions Wikiversity
B Iron changes into copper.
. In this reaction iron is reduced and copper is oxidized. In this reaction iron is reduced and copper is oxidized In this reaction both iron and copper are oxidized Which statement best describes the following reaction. Because of this in many cases H 2 O or a fragment of an H 2 O molecule H or OH in particular can participate in the redox reactionAs such we need to learn how to incorporate the solvent into a balanced redox equation.
Its atomic number is 14 and its atomic mass is 28. A chemical reaction in which electrons are released from the system. A chemical reaction in which electrons are transferred between reactants 2Beryllium Be has four electrons.
If there is formation of a precipitate the reaction is an oxidation-reduction reaction. Which statement best describes the following reaction. A chemical reaction in which electrons are released from the system D.
Nataly_w 17 1 year ago. Which statement best describes an endothermic reaction. Construct the redox reaction equation from the two half-reactions and calculate the cell potential from the standard potentials and predict if the reaction is reactant or product favoured.
The catalyst in a chemical equation is shown. 1Which statement best describes an oxidation-reduction reaction. B A reaction in which energy is released by the system.
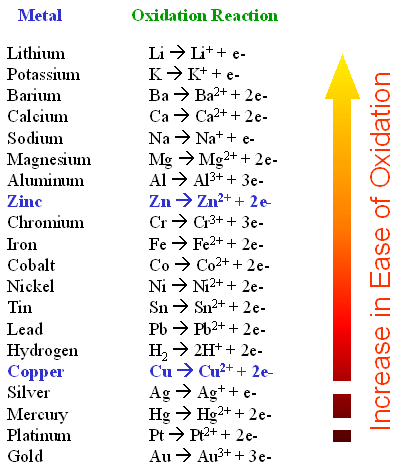
KU 1 a A reaction in which energy is absorbed by the system. MnO4 aq 8H aq 5e Mn2 aq 4H2O I E 151 V Sn2 aq Sn4 aq 2e E 015 V. Which of the following is true about a redox reaction.
In redox reactions a reduced half and an oxidized half occur together. How many atom in protons. Alex Ar 27 Answer.
E Two electrons are gained. Which equation represents the half-reaction that takes place at. D Copper transfers two electrons to iron.
KU 1 a on the reactant side. This is the basis of redox reactions. Two half-reactions of an electrochemical cell are given below.
An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule. These reactions involving electron transfers are known as oxidation-reduction or redox reactions. This is not an oxidation-reduction reaction.
Mg Mg2 2e D. The reaction that takes place in a chemical cell is best classi ed as A. Which answer best describes what is happening in the following reaction.
In this reaction iron is oxidized and copper is reduced. When an object is electroplated the occurrence of a redox reaction is nonspontaneous and it requires an electric current. D None of these apply.
The most common isotope of uranium has 92. A chemical reaction that involves oxygen C. 1Which statement best describes an oxidation-reduction reaction.
Fe Cu2 Fe2 Cu a. In this reaction both iron and copper are reduced. Third option is the correct one.
When an element is oxidized it loses electrons. This is a redox reaction in which octane C8H18 is oxidized. A chemical reaction in which there are fewer products than reactants.
C Iron transfers two electrons to copper. 4Al 302 2Al2O3 In this reaction aluminum is oxidized and oxygen is reduced In this reaction aluminum is reduced and oxygen is oxidized In this reaction th aluminum and oxygen are oxidized This is not an. But when an element is reduced it gains electrons.
Fe Cu2 Fe2 Cu A Two electrons are lost. However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons. In a redox reaction an electron is lost by the reducing agent.
In the reaction MgCl2MgCl2 the correct half-reaction for the oxidation that occurs is A. 1Which statement best describes an oxidation-reduction reaction1 point Chemistry. Cl2 2e 2Cl C.
Chemistry 19092021 2310 NetherisIsTheQueen. A chemical reaction in which there are fewer products than reactants B. O O O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction If there is formation of salt and water the reaction is an oxidation-reduction reaction.
An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species. The presence of which reactant is the best indicator of an oxidation-reduction reaction. C A reaction in which heat is released by the system.
A chemical reaction that involves oxygen. Which statement best describes what changes occur over the course of the following oxidation-reduction reaction. In this reaction both iron and copper are oxidized.

1 Which Statement Best Describes An Oxidation Reduction Reaction 1 Point A A Chemical Reaction In Brainly Com
No comments for "Which Statement Best Describes an Oxidation Reduction Reaction"
Post a Comment